Sponsors and Industry FAQs
Gold Coast Health is dedicated to the management and coordination of Clinical Trials and supports clinicians to undertake clinical research throughout our health service.
We recommend using our contact support centre to connect with our Clinical Trial Unit.
Yes, the Clinical Trial Unit is part of the Office of Research Governance and Development at Gold Coast Health. It is dedicated to the management and coordination of clinical trials, supporting clinicians throughout Gold Coast Health.
The Clinical Trial Unit facilitates the delivery of device and pharmaceutical clinical trials in each phase.
The team includes:
- Principal Investigators
- Associate Investigators
- Clinical Trial Manager - responsible for the operational activity of the Clinical Trial Unit, budgets and contracts
- Clinical Nurse - Senior Clinical Trial Coordinators - responsible for study start up activity, education and oversight of clinical trial activity
- Clinical Nurse - Clinical Trial Coordinators - responsible for coordinating and delivering Clinical Trials
- Registered Nurse - Clinical Trials
The Clinical Trial Unit works with both internal collaborators such as Pharmacy, Imaging, Pathology and external services to deliver our clinical trials.
Each member of the Clinical Trial Unit receives clinical trial training, education and support to deliver high quality clinical trials. In addition, each member has a valid Good Clinical Practice refreshed every two years and a current research CV updated two yearly.
Whilst the clinical team provides an around the clock service for urgent participant safety enquiries, the Clinical Trial Unit currently operates a weekday only service.
Gold Coast Hospital and Health Service (GCHHS) offers Good Clinical Practice (GCP) compliant facilities and resources expected from Clinical Trial sites. Learn more about what we have to offer.
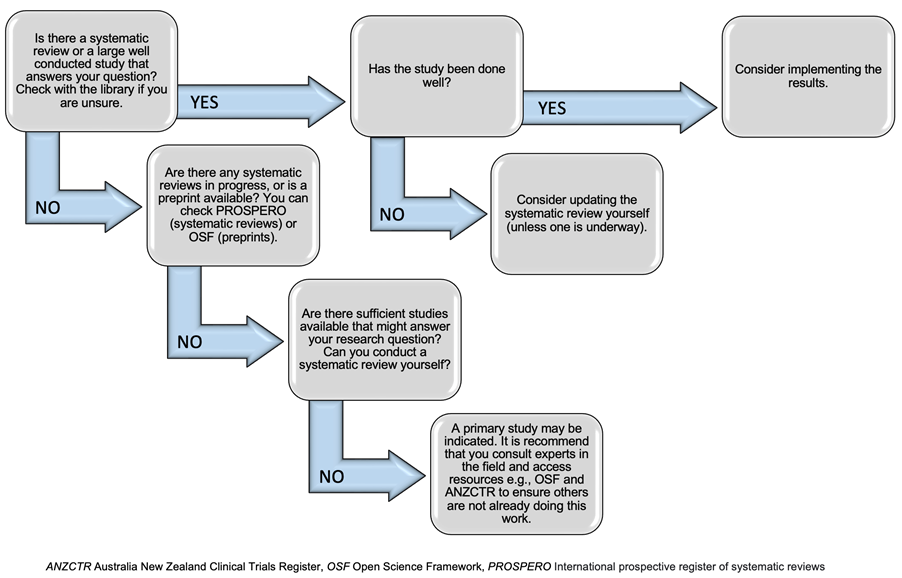
Clinical Trial related Procedures are currently under review and development to ensure compliance with the Clinical Trials Governance Framework. However, the Research Policy at Gold Coast Health endorses the current use of the National Clinical Trial Standard Operating Procedures (SOPs).
The Clinical Trial Manager can advise the current timelines for study start up activity, but the guide below provides an estimation. Please note all Clinical Trials require at least 16 weeks to process through the stages, however this can sometimes be much longer depending on the requirements.
Gold Coast Health currently operates Ethical Review Manager (ERM) to correspond with Human Research Ethics Committee (HREC) and Research Governance.
Feasibility: Protocol review with Clinical Team and completion of Feasibility Questionnaire 2-4 weeks
Site Selection: Meet the team and Site Qualification Visit 2-3 weeks
Pre Site-Selection: Pre Start Up Agreement 1-2 weeks
HREC Submission: 6-8 weeks from application to initial feedback (if Lead Site). Gold Coast Health can only accept HREC approval from an NMA listed HREC
Pre Governance Submission: 5 weeks, if QCAT or PHA approval is required a further 3 weeks should be anticipated
Governance Review: 4-6 weeks
Post Governance Authorisation: Staff Training and SIV 2 weeks - SITE ACTIVATION